نظرا لأن المزيد والمزيد من الناس بحاجة إلى فهم صناعة تخزين الطاقة ، والبطاريات هي جوهر تخزين الطاقة ، فقد جمعت Qingzhou Technology المعلومات ذات الصلة وجمعت معلومات بسيطة ومتعمقة ليتعلمها الجميع. أعتقد أنه بعد قراءته ، ستشكل إطارا معرفيا أكثر احترافا للبطاريات وتصبح نصف خبير. أتمنى لك دراسة سعيدة ~
1. عائلة البطارية
Table of Contents
Toggle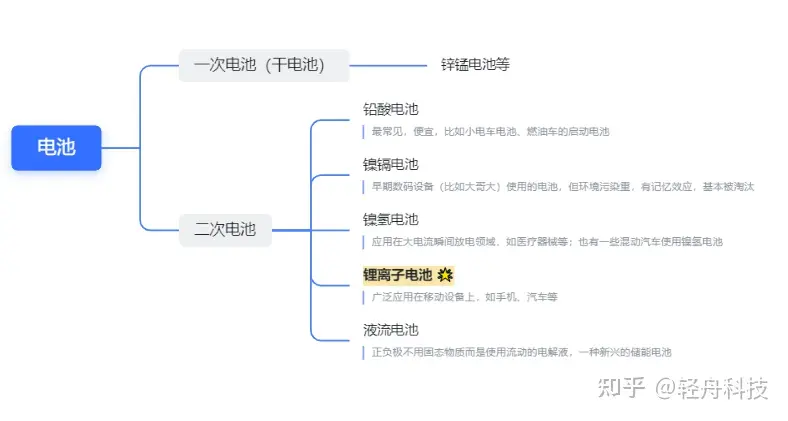
* يشير اسم “بطارية الليثيوم” في الأصل إلى بطارية ليثيوم معدنية ، وهي بطارية أساسية ، لكنها لم تعد تستخدم لأنها متفجرة. ما يسمى بطاريات الليثيوم هي عموما بطاريات ليثيوم أيون.
البطاريات 7 و 5 التي نستخدمها عادة كلها بطاريات جافة. استخدم “الأخ الأكبر” للهاتف المحمول من الطوب في السنوات الأولى بطاريات هيدريد معدن النيكل ؛ عادة ما تكون البطاريات المستخدمة في الترام الصغيرة بطاريات الرصاص الحمضية ، ويتم تعبئة مجموعة من 4 بشكل مباشر. تستخدم بطاريات الليثيوم أيون في الغالب في هواتفنا المحمولة وأجهزة الكمبيوتر المحمولة وحتى السيارات الكهربائية.
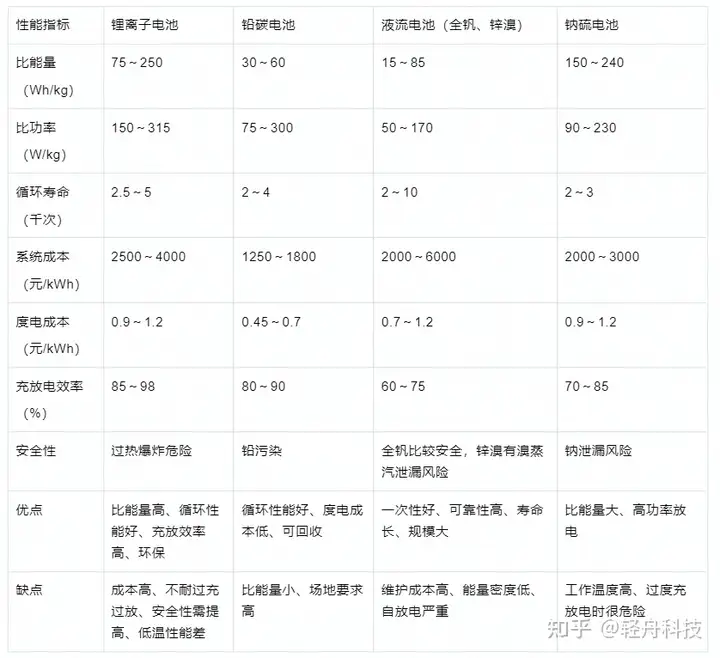
مقارنة بين خصائص بطاريات تخزين طاقة البطارية الكهروكيميائية الرئيسية
2. تحليل اسم البطارية
SOX: الاسم الكامل هو State Of X ، وصف حالة البطارية ، H هو Health باللغة الإنجليزية ، C هي السعة ، P هي الطاقة ، E هي الطاقة ، تشبه إلى حد ما معلمات المحرك ، الإزاحة ، الطاقة ، الطاقة ، وقت التشغيل ، إلخ. تقريبا نفس الشيء.
SOC:(State of Charge) refers to the charge capacity of the battery. The power of the battery is like the water in the bucket. How much available power is in the battery at a certain moment is called the SOC of the battery at that moment. When the battery is completely discharged, the SOC is 0, and when it is fully charged The lower SOC is 1. Usable capacity/actual capacity.
DOD: (Depth of Discharge) refers to the discharge depth of the battery. When the battery is fully charged, its DOD is 0, and when it is completely discharged, its DOD is 1. Therefore, the DOD of the battery under normal conditions is a value between 0 and 1, and the DOD and SOC The relationship is: DOD+SOC=1.
SOH:(State of health) refers to the current actual capacity of the battery/the initial rated capacity of the battery. As the battery ages, the SOH will continue to decrease. Generally measured according to capacity and internal resistance.
The literature on SOH defined by battery capacity fading is the most, and the definition of SOH given is as follows:
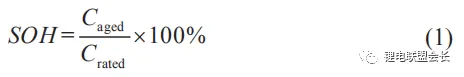
In the formula: Caged is the current capacity of the battery; Crated is the rated capacity of the battery.
3. Lithium battery classification
Sorted by practical performance: power type (sprint, short-term high power output) and energy type (long-distance running, high energy storage)
Sorted by appearance: cylindrical, square (steel/aluminum shell), soft package (aluminum-plastic film)
Sorted by electrolyte material: liquid lithium-ion battery (LIB) and polymer lithium-ion battery (PLB).
Liquid lithium-ion batteries use liquid electrolytes (currently most power batteries are of this type). Polymer lithium-ion batteries are replaced by solid polymer electrolytes, which can be “dry” or “colloidal”. At present, most of them use polymer gel electrolytes. Regarding solid-state batteries, it strictly means that both electrodes and electrolytes are solid.
Sorted by positive electrode material: lithium iron phosphate battery (LFP), lithium cobalt oxide battery (LCO), lithium manganese oxide battery (LMO), (binary battery: lithium nickel manganese oxide/lithium nickel cobalt oxide), (ternary: nickel cobalt oxide Lithium manganese oxide battery (NCM), lithium nickel cobalt aluminate battery (NCA)).
Sorted by negative electrode material: lithium titanate battery (LTO), graphene battery, nano carbon fiber battery.
18650 battery
18650 is a type of lithium-ion battery, similar to dry batteries No. 7, No. 5, No. 1, etc.
18650 is the originator of lithium-ion batteries – a standard lithium-ion battery model set by Japan’s SONY company in order to save costs. 18 means a diameter of 18mm, 65 means a length of 65mm, and 0 means a cylindrical battery. Common 18650 batteries are divided into lithium-ion batteries and lithium iron phosphate batteries. The nominal voltage of lithium-ion batteries is 3.7v, the charging cut-off voltage is 4.2v, and the capacity is usually 1200mAh-3350mAh. The nominal voltage of lithium iron phosphate batteries is 3.2V, the charging cut-off voltage is 3.6v, and the common capacity is 2200mAh-2600mAh .
Advantages of 18650:
Standardization: 18650 battery is the earliest, most mature, and most stable lithium-ion battery. Japanese manufacturers have accumulated a high level of consistency. It is easy to replace a single battery when there is a problem.
Safety: 18650 batteries are generally steel shells, which are safer than possible collision problems; and with the continuous improvement of the production process level of 18650 batteries, the safety is also constantly improving. Steel shell lithium batteries are prone to explosion problems, but now 18650 batteries are designed with safety valves, which can not only release excessive internal pressure, but also physically disconnect the battery from the external circuit, which is equivalent to physically isolating the battery. To ensure the safety of other batteries in the battery pack.
However, other batteries also have certain advantages compared with them. For example, polymer lithium batteries have high relative safety, high energy density, low packaging film strength, and will not explode. The worst is burning. The design can be customized, but the corresponding research and development costs increase and the versatility decreases.
Lithium iron phosphate battery (LFP)
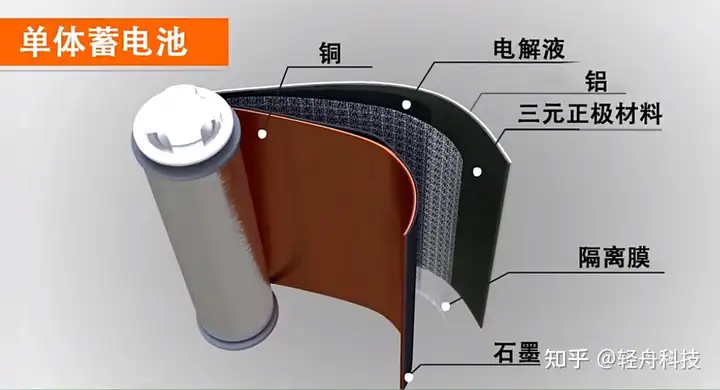
Lithium iron phosphate (molecular formula LiFePO4, Lithium Iron Phosphate, also known as lithium iron phosphate, lithium iron phosphorus, referred to as LFP), is a positive electrode material for lithium ion batteries, also known as lithium iron phosphorus batteries. Elements, raw material prices are low and phosphorus, lithium, and iron are abundant in the earth’s resources, so there will be no supply problems. It has moderate working voltage (3.2V), large capacity (170mAh/g), high discharge power, fast charging and long cycle life, and high stability in high temperature and high heat environment.
4. Lithium battery voltage, capacity
The voltage of a lithium-ion battery will vary with the discharge current, ambient temperature, and positive and negative electrode materials.
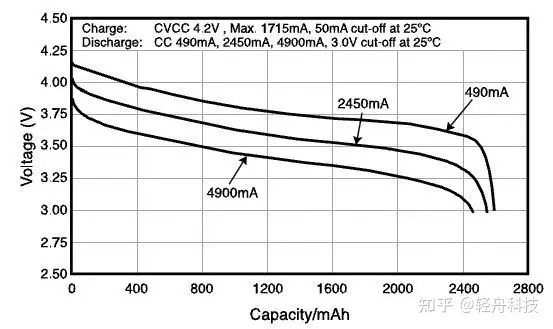
This picture is the discharge curve of a Panasonic 2550mAh lithium-ion battery using lithium cobaltate as the positive electrode material. The three curves from top to bottom represent the changes in voltage and capacity when using three different discharge currents.
First of all, in the process of charging and discharging, the voltage is continuously changing. Taking 490mA as an example, when the battery is fully charged, the open circuit voltage is 4.2V. As the discharge progresses, the voltage (ordinate) decreases slowly, and the discharged power (abscissa) gradually increases until the voltage begins to drop sharply at 3.5V. Although the voltage has been changing throughout the discharge process, for the sake of simplicity, only the average value of 3.7V in the gentle discharge part of the curve is marked as the battery voltage. This voltage is also called the nominal voltage.
The voltage is measured under low current and room temperature environment, and it will decrease with the increase of discharge current and decrease of temperature.
Another important factor affecting the battery voltage is the positive and negative electrode materials. The aforementioned Panasonic batteries use lithium cobalt oxide and graphite as the positive and negative electrode materials, which were also the standard materials for the entire lithium battery industry in the past few years. With the application of new materials in batteries, some 3.6V or 3.8V lithium batteries have appeared in the past two years. They use different positive electrode materials. Compared with lithium cobalt oxide batteries, they can all increase the energy density, that is, in the unit Store more power in weight and volume.

The battery capacity is divided into rated capacity and actual capacity.
1. The actual capacity refers to the amount of electricity actually released by the battery under certain discharge conditions. Actual capacity is always lower than theoretical capacity.
2. Rated capacity refers to the minimum amount of electricity that the battery should discharge under certain discharge conditions when designing and manufacturing the battery.
The battery capacity is generally calculated in AH (ampere hours, ampere hours), and the single battery is generally marked as mAh (milliampere hours) for convenience. If the rated capacity of the battery is 1300mAh, that is, a current of 130mA is used to discharge the battery, then the battery can continue to work for 10 hours (1300mAh/130mA=10h). This is an analysis in an ideal state. The current of a digital device cannot always be constant at a certain value when it is actually working.
The capacity of 18650 lithium battery is generally between 1200mah~3600mah.
Now the unit to measure the battery capacity of a mobile phone is mAh. High school knowledge tells us that this is the unit of power, and it needs to be multiplied by a voltage to be the unit of energy.
Battery capacity calculation method:
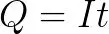
Battery energy calculation method:
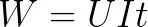
the mobile phone consumes energy, so how to measure it with a unit of power? ! This is actually because in portable electronic devices such as mobile phones, in order to reduce the volume of the battery, the positive electrode of the lithium-ion battery uses lithium cobalt oxide, which has a high compaction density. Because the positive electrodes of mobile phone batteries are all made of the same material, the voltage of the batteries is relatively close (theoretically 3.7V, which is related to the technology of different battery manufacturers), and there is often only one single cell in the mobile phone. Batteries (please ignore a certain mobile phone produced by a mobile phone factory recently, which has two 2000mAh batteries), the capacity can actually measure the amount of energy stored in the battery.
But the battery on the computer will mark the capacity (power) and energy of the battery at the same time. This is because there is not only one single battery in the computer, but also many batteries connected in series and parallel to form a battery pack, so the capacity of the battery cannot be measured. High school knowledge tells us: for the same type of battery, after the batteries are connected in parallel, the voltage of the battery pack will not change, but the capacity of the battery pack will increase; if the batteries are connected in series, the capacity of the battery pack will remain the same, but the voltage of the battery pack will increase.
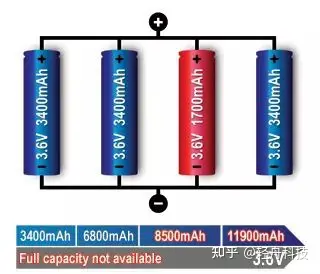

Then I went to see the battery specifications of another laptop, as shown in the picture below.

At first glance, the capacity of this battery is a little larger than that of a mobile phone. How can it be used for a laptop? But looking at its voltage, it suddenly dawned on me. This is composed of six batteries with a capacity of 2200mAh and a voltage of 3.6V connected in series and parallel, which is the legendary 6-cell lithium-ion battery (there are 6 single batteries inside). From the shape of this battery (long) and the capacity of 2200mAh, it can be seen that this is an 18650 battery (that is, a cylindrical battery with a diameter of 18mm and a height of 65mm).
Therefore, to measure the battery life, it is more reliable to look at the energy, and do the math yourself.
5. Why Choose Li-ion Batteries
Light
The weight energy density of lithium-ion batteries is generally 200-260wh/g; lead-acid batteries are generally 50-70wh/g, and nickel-hydrogen batteries are 40-70wh/kg. This means that under the same capacity, the other two batteries are 3 to 5 times heavier than lithium batteries. Therefore, in terms of lightweight energy storage devices, lithium batteries occupy an absolute advantage.
The volume capacity density of lithium batteries is usually about 1.5 times that of lead-acid batteries, and the energy density of nickel-metal hydride batteries is only 60-80% of lithium-ion batteries, so under the same capacity, the volume of lithium batteries is also smaller.
Fast charging
Due to the active performance of lithium ions, the speed of moving inside the battery is relatively fast, so the charging current is large and the charging speed is fast. A lithium ion battery can be fully charged in about 3 hours; while the charging speed of Ni-MH batteries is very slow, it takes about 3 hours to fully charge. 1 day.
no memory effect
The memory effect is an effect in which the battery contents crystallize due to use. For nickel-based batteries, the crystals on the nickel plate will become coarse after a long time, affecting the contact with the electrolyte, resulting in a decrease in capacity. Performing several complete charge and discharge can refine the crystal grains and partially restore the capacity, which is the so-called “activation”.
The lithium battery has no memory effect, and only needs 3-5 normal charge and discharge cycles to activate the battery and restore the normal capacity.
Environmental friendly
Under the environmental protection policy, in order to ensure that the environment in which it lives can reduce pollution, there are pollutions caused by improper handling in the production, use and recycling of lead-acid batteries, while lithium batteries have relatively perfect packaging and sealing work. It will be relatively environmentally friendly.
6. Safety issues of lithium batteries
Lithium batteries have the advantages of light weight and fast charging, so why are other secondary batteries such as lead-acid batteries still in circulation in the market?
In addition to issues such as cost and different fields of application, another reason is safety.
Lithium is the most active metal in the world. Due to its chemical properties are too active, when lithium metal is exposed to the air, it will have a violent oxidation reaction with oxygen, so it is prone to explosion, combustion and other phenomena. In addition, during the charging and discharging process of lithium batteries, redox reactions will also occur inside. The explosion and spontaneous combustion are mainly caused by the accumulation of lithium batteries after heating, and there is no time for diffusion and release. To put it simply, a lithium battery will generate a lot of heat during charging and discharging, which will cause the internal temperature of the battery to rise and the temperature between the single cells to be uneven, resulting in unstable performance of the battery.
thermal runaway
Unsafe behavior of lithium-ion batteries (including battery overcharge and overdischarge, rapid charge and discharge, short circuit, mechanical abuse conditions and high temperature thermal shock, etc.) can easily trigger dangerous side reactions inside the battery to generate heat, directly destroying the negative and positive electrodes passivation film on the surface.
There are many reasons for the triggering of lithium-ion battery thermal runaway accidents. According to the characteristics of the triggering, it can be divided into three types: mechanical abuse triggering, electrical abuse triggering and thermal abuse triggering.
Mechanical abuse: refers to acupuncture, extrusion, and heavy impact caused by car collisions, etc.;
Electrical abuse: generally caused by improper voltage management or electrical component failure, including short circuit, overcharge and overdischarge, etc.;
Thermal Abuse: Caused by overheating due to improper temperature management.
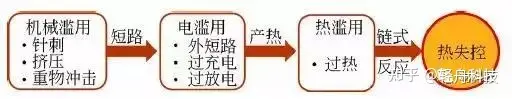
These three triggering methods are interrelated. As shown in the figure above, mechanical abuse will generally cause deformation or rupture of the battery diaphragm, resulting in direct contact between the positive and negative electrodes inside the battery and short circuit, resulting in electrical abuse; and under electrical abuse, Joule heat and other products The increase of heat causes the temperature of the battery to rise, which develops into heat abuse, which further triggers the chain heat generation side reaction inside the battery, and finally leads to the thermal runaway of the battery.
The thermal runaway of the battery is caused by the fact that the heat generation rate of the battery is much higher than the heat dissipation rate, and a large amount of heat is accumulated and not dissipated in time. In essence, “thermal runaway” is an energy positive feedback cycle process: increasing temperature causes the system to heat up, the system heats up, and the temperature rises, which in turn makes the system hotter.
The process of thermal runaway:
The internal temperature of the battery rises, and the SEI film on the surface of the SEI film decomposes at high temperature, and the lithium ions embedded in the graphite will react with the electrolyte and binder, further pushing the battery temperature up to 150°C, and there is a new violent heat release at this temperature The reaction happens.
When the battery temperature reaches above 200°C, the positive electrode material decomposes, releasing a large amount of heat and gas, and the battery starts to bulge and continues to heat up. At 250-350°C, the lithium-intercalated negative electrode begins to react with the electrolyte.
The positive electrode material in the charged state begins to undergo a violent decomposition reaction, and the electrolyte undergoes a violent oxidation reaction, releasing a large amount of heat, generating high temperature and a large amount of gas, and the battery burns and explodes.
Overcharge
The problem of lithium dendrite precipitation during overcharging:
Lithium cobalt oxide batteries, after being fully charged, still have a large amount of lithium ions remaining on the positive electrode. That is to say, the negative electrode cannot accommodate more lithium ions attached to the positive electrode, but in the overcharged state, the excess lithium ions on the positive electrode will still swim to the negative electrode, and metal oxides will form on the negative electrode because they cannot be fully accommodated. Lithium, because this metal lithium is a dendritic crystal, it is called a dendrite. When the dendrite is too long, it is easy to pierce the separator and cause an internal short circuit. Since the main component of the electrolyte is carbonate, its ignition point and boiling point are low, so it will burn or even explode when the temperature is high.
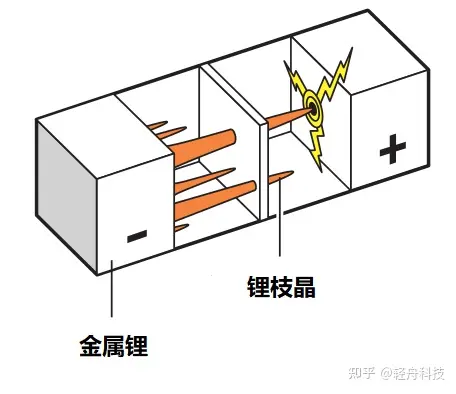
If it is a polymer lithium battery, the electrolyte is a colloid, which is prone to more violent combustion.
To solve this, scientists try to replace safer cathode materials. The material of lithium manganese oxide battery has certain advantages. It can ensure that the lithium ions of the positive electrode can be completely embedded in the carbon pores of the negative electrode in the fully charged state, instead of leaving certain residues in the positive electrode like lithium cobalt oxide. To a certain extent The formation of dendrites is avoided. The stable structure of lithium manganese oxide makes its oxidation performance far lower than that of lithium cobalt oxide. Even if it is short-circuited externally (rather than internally), it can basically avoid the precipitation of metallic lithium and cause combustion and explosion. Lithium iron phosphate has higher thermal stability and low electrolyte oxidation ability, so it has high safety.
Ageing
The aging attenuation of lithium-ion batteries is externally manifested as capacity attenuation and internal resistance increase, and its internal aging attenuation mechanism includes the loss of positive and negative active materials and the loss of available lithium ions.
When the negative electrode material is aging and attenuated, and the negative electrode capacity is insufficient, the risk of lithium precipitation at the negative electrode is also more likely to occur.
In the case of over-discharge, etc., the potential of the negative electrode to lithium will rise to more than 3V, which is higher than the dissolution potential of copper, resulting in the dissolution of the copper current collector. The dissolved copper ions will precipitate on the positive electrode surface and form copper dendrites. Copper dendrites will pass through the separator, causing an internal short circuit and seriously affecting the safety performance of the battery.
In addition, the overcharge resistance of aging batteries will decline to a certain extent, mainly due to the increase in internal resistance and the decrease in positive and negative active materials, resulting in increased Joule heat during battery overcharging, which may trigger side effects with less overcharging. reaction, causing thermal runaway of the battery. In terms of thermal stability, lithium precipitation at the negative electrode will lead to a sharp decline in the thermal stability of the battery.
All in all, the safety performance of the aging battery will be greatly reduced, seriously endangering the safety of the battery.
Solution
The most common solution is to equip the battery energy storage system with a battery management system (BMS). For example, the 8,000 18650 batteries used in the Tesla Model S use its battery management system to monitor various physical parameters of the battery in real time and evaluate the battery’s service status. , and online diagnosis and early warning, but also discharge and precharge control, battery balance management and thermal management, etc.
7. Lithium battery application
At this stage, lithium iron phosphate (LFP) batteries are mostly used in lithium battery energy storage systems. Lithium iron phosphate batteries have a series of unique advantages such as high working voltage, high energy density, long cycle life, and environmental protection.
Ternary Lithium Battery (Ternary Lithium Battery) refers to a lithium battery that uses nickel-cobalt lithium manganate or nickel-cobalt lithium aluminate as the positive electrode material, graphite as the negative electrode material, and lithium hexafluorophosphate-based lithium salt as the electrolyte. Because its positive electrode material contains three metal elements: nickel, cobalt, and manganese/aluminum, it is named “ternary”. Ternary lithium batteries are mainly used in the field of power batteries.
https://zhuanlan.zhihu.com/p/401504245
Related Posts
- هيكل وإيجابيات وسلبيات بطاريات الليثيوم المربعة
- هل يجب أن أترك عاكس RV قيد التشغيل عند توصيله بالكهرباء؟
- مكان التخلص من بطاريات الليثيوم أيون بشكل آمن: دليلك المحلي
- مقدمة لبطاريات الليثيوم أيون الثلاثية
- معلمات اختبار الأداء لبطاريات الليثيوم
- ما هو عدد الدورات والعمر الحقيقي لحزم بطاريات ليثيوم فوسفات الحديد؟


































